In vivo toxicology is a branch of toxicology that studies the effects of chemicals and other substances on living organisms. It is used to evaluate the potential adverse effects of drugs, industrial chemicals, food additives, cosmetic ingredients, and other substances on humans, animals, and the environment. It involves laboratory studies of the effects of a substance on living organisms, such as rats, rabbits, and monkeys, as well as studies of the effects of a substance on humans. In vivo toxicology studies may evaluate the acute toxicity of a substance, its effects on the development and reproductive systems, its chronic toxicity, and its potential to cause cancer.
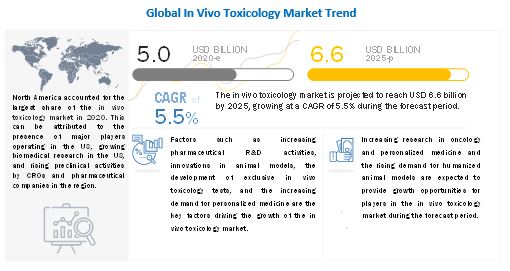
Currently, in vivo toxicology market size is projected to reach USD 6.6 billion by 2025 from USD 5.0 billion in 2020, at a CAGR of 5.5% during the forecast period. Key attributing factors contributing to the growth of global market include development of innovative animal models and expanding competition in the in vivo toxicology testing market space. However, stringent laws and regulations pertaining to use of animal models in research is expected to emerge as a key challenge to this market.
Request for assumptions & how numbers were triangulated.
https://www.marketsandmarkets.com/requestsampleNew.asp?id=105308811
Market Drivers
- Increasing pharmaceutical R&D activities
- Innovations in animal models
- Exclusive in vivo toxicology tests
- Increasing demand for personalized medicine
Market Opportunities
- Rising demand for humanized animal models
- Expansion of Toxicological Testing Services
Market Challenges
- Regulations and laws for the ethical use of animals in research
- Shortage of Qualified Toxicologists
The prominent players operating in the in vivo toxicology market are Charles River Laboratories (US), The Jackson Laboratory (US),Envigo (US), Taconic Biosciences, Inc. (US), and JANVIER LABS (France), Thermo Fisher Scientific (US), Danaher Corporation (US), Waters Corporation (US), Agilent Technologies (US), Shimadzu Corporation (Japan), Bruker Corporation (US), PerkinElmer (US). Other prominent players includeMerck KGaA (Germany), GE Healthcare (US), and Bio-Rad Laboratories (US), genOway (France), Cyagen Biosciences (US), GVK BIO (India), PolyGene (Switzerland), Crown Biosciences (US), TransCurebioServices (France), Ozgene Pty Ltd. (Australia), Harbour BioMed (US)
Charles River Laboratories International, Inc. (US)
Charles River has extensive portfolio of animal models, particularly mice models and services. With more than 70 years of experience, the company has built upon its core competency in the field of in vivo biology through its diverse products and services portfolio. It offers products, services, and solutions that focus specifically on early-stage drug discovery and preclinical development. Its strong portfolio enables it to increase collaboration with clients, from early lead generation to candidate selection. Charles River Laboratories has nearly 90 facilities spread across 20 countries. The company has its presence in the US, Canada, the UK, France, Italy, Spain, the Netherlands, Belgium, Germany, Poland, Ireland, Finland, Luxembourg, Japan, China, India, South Korea, Hong Kong, and Australia.
The Jackson Laboratory (US)
The Jackson Laboratory has a portfolio of over 11,000 mice strains. In 2019, the company distributed 3 million JAX mice to approximately 1,400 organizations in 50 countries. The company not only acts as a distributor but also focuses on in-house innovative mice model development. For instance, in February 2020, the company launched the unique K18-hACE2 transgenic mouse model for coronavirus research. The company is utilizing state-of-the-art breeding techniques to grow a new K18-hACE2 transgenic mouse colony rapidly. The company has received many grants, which supports the development of its mice model business. Over the last few years, the company has received grants/funds from leading health organizations and academic institutes such as the National Cancer Institute (NCI), National Institutes of Health (NIH), The Mark Foundation for Cancer Research, National Institute on Deafness and Other Communication Disorders, National Institute of Neurological Disorders and Stroke, and Howard Hughes Medical Institute (HHMI) (US), among others. These grants have helped the company to develop new models, conduct specific studies on the models, compile genome-scale datasets for mice and humans, and examine various patterns of gene expression in mice to gain a better understanding of the genetic programs responsible for normal development and disease.
Envigo (US)
The company has built upon its core competency in preclinical and non-clinical contract research, research models and services, and animal care products. It offers a wide range of rodent models and services. Envigo has a strong focus on the developing Asian market. Harlan Laboratories (now a part of Envigo) was the first international player to enter the Indian mice model and services market. This has helped the company to establish relationships with several leading life sciences researchers in India, including pharmaceutical, biotechnology, and biopharmaceutical companies, CROs, and private and government research institutes. In January 2014, Harlan Laboratories expanded its presence in India with the introduction of a new research model breeding facility located in Hyderabad, thereby helping it to better serve the company’s current customers.
Download an Illustrative Overview:
https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=105308811
Report Objectives
- To define, describe, and measure the global in vivo toxicology market based on product, test type, testing facility, toxicity endpoint, end user, and region
- To provide detailed information regarding the major factors, such as drivers, restraints, opportunities, and challenges, influencing the growth of the market
- To strategically analyze the micromarkets1 with respect to individual growth trends, prospects, and contributions to the global in vivo toxicology market
- To analyze the opportunities in the global in vivo toxicology market for key stakeholders and provide details of the competitive landscape for major market leaders
- To forecast the size of the market segments with respect to five main regions: North America, Europe, Asia Pacific, Latin America and Middle East & Africa
- To profile the key players and comprehensively analyze their core competencies2 in terms of key developments, product portfolios, and financials
- To track and analyze competitive developments such as partnerships, agreements, alliances, joint ventures, collaborations, product launches, expansions, acquisitions, grants/funds, and licensing agreements, among others, in the in vivo toxicology market
Recent Developments
- In 2020, GenOway acquired exclusive worldwide rights from Merck for its foundational CRISPR/Cas9 portfolio in the rodent field.
- In 2020, Taconic entered into an agreement with the University of Texas Medical Branch to distribute humanized ACE2 mice for COVID-19 research.
- In 2020, The Jackson Laboratory started the production of ACE2 mice to support the research on COVID-19.
- In 2020, Danaher Corporation acquired GE Healthcare’s Life Sciences business.
- In 2020, Thermo Fisher launched Orbitrap Exploris 240 and Orbitrap Exploris 120 mass spectrometers.
- In 2020, Waters Corporation announced the establishment of Immerse Cambridge, a research laboratory in the heart of Cambridges Kendall Square. Immerse Cambridge will serve as a strategic, collaborative space in the community, where Waters can partner with academia and research to accelerate the next generation of scientific advancements.
Content Source:
https://www.marketsandmarkets.com/Market-Reports/in-vivo-toxicology-testing-market-105308811.html
https://www.marketsandmarkets.com/PressReleases/in-vivo-toxicology-testing.asp